We will send you an email to reset your password.
Couldn't load pickup availability
Product Name
Saliva Glucose Testing (Double Enzyme Method)
Packaging Specification
1 tests per box
Intended Use
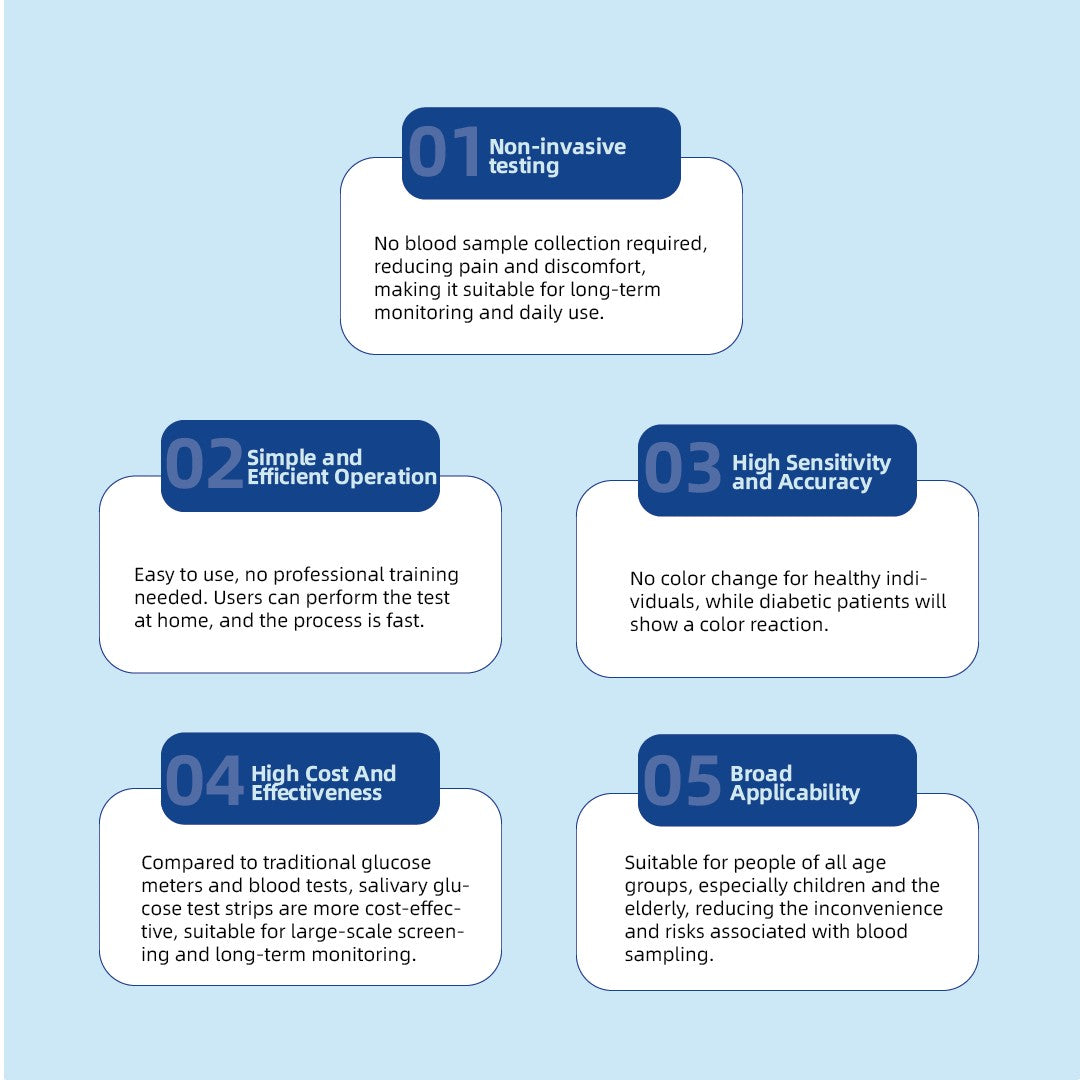
This product is designed for the qualitative detection of glucose levels in human saliva for in vitro diagnostic use.
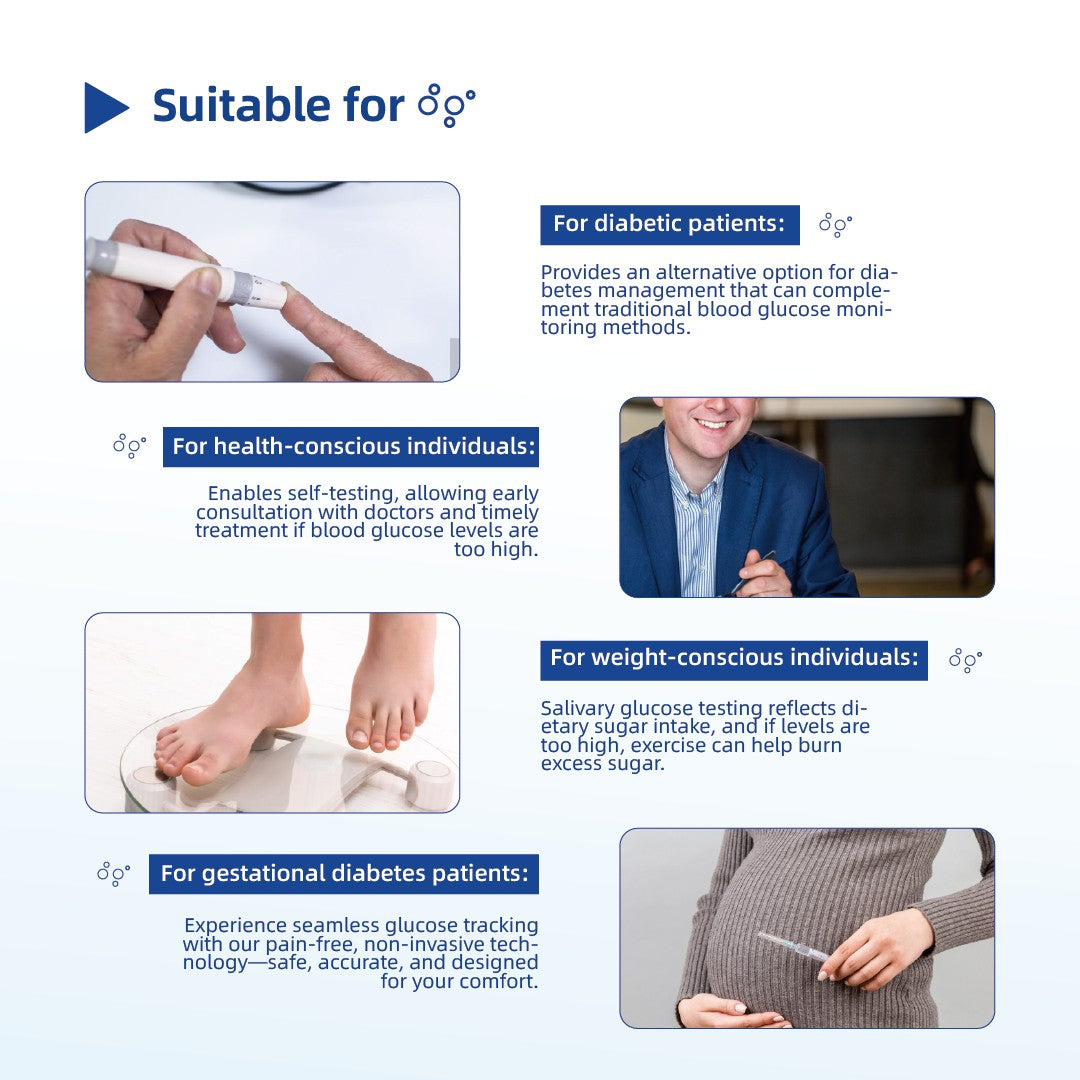
Numerous studies have shown that elevated glucose levels in saliva can have significant negative effects on human health. On one hand, high glucose content in saliva can disrupt the oral environment, increasing the osmotic pressure, lowering the pH, and slowing down the salivation rate, thereby weakening the natural cleansing ability of saliva. On the other hand, elevated glucose levels provide nutrients for the growth of microbial communities, such as Streptococcus mutans, Candida, and Lactobacillus, leading to the proliferation of oral microorganisms. These two factors together contribute to oral conditions such as dry mouth, periodontitis, gingivitis, and tooth decay. In particular, individuals with diabetes, who tend to have higher glucose levels in their saliva, are at a significantly higher risk of developing oral diseases.
Common clinical or laboratory diagnostic methods include: glucose oxidase method, ion chromatography, and liquid chromatography.
Principle of the Test
Under the catalysis of glucose oxidase (GOD), glucose in the sample reacts with oxygen to produce hydrogen peroxide. Then, under the action of horseradish peroxidase (HRP), the hydrogen peroxide reacts with a chromogenic reagent to produce a red-colored imine compound. The amount of the red compound formed is directly proportional to the glucose concentration, and thus, the color of the reaction product can indirectly reflect the glucose content.
Key Ingredients
|
Index |
Item |
Quantity |
Composition |
|
|
Glucose Test Strips |
1 test/box |
Enzyme-loaded filter paper mounted on PET plastic backing |
|
|
Main Components |
/ |
R1: Reagent 1 (Glucose Oxidase 10 mg, Horseradish Peroxidase 7.5 mg); |
|
|
Instruction Manual |
1 |
/ |
Storage Conditions and Shelf Life
1. This product has a shelf life of 6 months when stored at -15°C to -25°C.
2. Once the aluminum foil bag is opened, store it at 2–8°C and use within 1 hour.
3. The production date and expiration date can be found on the product label.
Sample Requirements
1. The sample required for this product is human saliva.
2. For sample collection, sit with your body naturally leaning slightly forward. Place the saliva collector below your mouth and collect approximately 1 mL of naturally secreted saliva. The test should be completed within 10 minutes at room temperature (10–30°C).
3. Sample Storage: Store at -80°C or in liquid nitrogen for no more than 2 days.
4. Transportation: Cold chain transport (liquid nitrogen, -196°C) for no more than 2 days.
Test Method
1. Rotate the saliva collector and remove the collection funnel.
2. Immerse one end of the saliva glucose test strip into the saliva for 30 seconds. After removal, observe the color with the naked eye. If the color has not changed within 2 minutes, the test result is invalid. Note: For accuracy, observe the test result in a well-lit area.
Interpretation of Test Results
1. If the test strip is white or light yellow, it indicates that the saliva glucose level is normal. If the test strip is yellow-red, orange-red, pink, red, or brown-red, it indicates that the saliva glucose level exceeds the normal range.

2. The optimal testing time is either on an empty stomach, before a meal, or 2 hours after a meal.
3. The test results remain unaffected when sample concentrations are within the following thresholds: vitamin C ≤10,000 mg/L, creatinine ≤10,000 mg/L, glutathione ≤10,000 mg/L, fructose ≤20 mg/L, maltose ≤200 mg/L, ribose ≤10,000 mg/L, lactose ≤1,000 mg/L, xylose ≤500 mg/L, sucrose ≤10,000 mg/L, mannose ≤100 mg/L, and galactose ≤1,000 mg/L. For samples containing 10 mg/L glucose, the interference thresholds are more stringent for certain substances (e.g., vitamin C ≤5 mg/L). These specifications ensure test accuracy under normal physiological conditions.
4. For samples containing 10 mg/L glucose, the following concentrations do not affect results: Vitamin C ≤ 5 mg/L, Creatinine ≤ 100 mg/L, Glutathione ≤ 10,000 mg/L, Fructose ≤ 20 mg/L, Maltose ≤ 200 mg/L, Ribose ≤ 10,000 mg/L, Lactose ≤ 1,000 mg/L, Xylose ≤ 500 mg/L, Sucrose ≤ 10,000 mg/L, Mannose ≤ 1,000 mg/L, Galactose ≤ 1,000 mg/L.
Positive Determination Values
1. After testing 175 saliva samples from subjects, it was found that 95% of healthy individuals have a saliva glucose concentration < 10 mg/L.
2. The positive determination value for this project refers to physiological biochemical parameters obtained under specific conditions using specific methods. The positive value range may vary based on conditions such as region, time, gender, age, living standards, and testing methods. It is recommended that users establish their own positive determination value range within their laboratory.
Limitations of the Testing Method
1. This test strip is for human saliva testing only.
2. The results of this test strip should only be used as a diagnostic aid. Test results should be considered alongside other clinical and experimental data. If the results do not match clinical evaluations, further tests are necessary.
3. Since test strips prepared using reagents from different manufacturers may produce different results when testing the same sample, test results should not be directly compared to avoid incorrect medical interpretation.
4. Other factors, such as technical issues, user errors, improper product storage, and sample factors, may also cause incorrect results.
5. Low-temperature conditions can affect the sensitivity of the color reaction. The test should be performed at a temperature range of 10-40°C.
Product Performance Indicators
1. Appearance: The test strip surface should be smooth and flat, without obvious protrusions, scratches, burrs, or other defects. The text and markings should be clear and reliable. Plastic parts should be free from bubbles, cracks, or deformation.
2. Strip Width: (1.0±0.1) cm.
3. Critical Value: Test 5 detection limit reference samples (L1-L5). The L1 result should be negative, L2 should be negative or positive, and L3-L5 should be positive.
4. Positive Reference Sample Conformity Rate: Test 3 positive reference samples (P1-P3). All results should be positive, with a conformity rate of 100%.
5. Negative Reference Sample Conformity Rate: Test 3 negative reference samples (N1-N3). All results should be negative, with a conformity rate of 100%.
6. Repeatability: Test 2 precision reference samples (J1 and J2) 10 times each. All results should be positive, with uniform color development.
7. Specificity: Test 2 specificity reference samples: creatinine 10 mg/L (S1) and glutathione 10 mg/L (S2). The results should both be negative.
8. Batch-to-Batch Variability: Take 3 batches of reagents and test precision reference samples (J1 and J2) 10 times each. The results should all be positive, with uniform color development.